ARUP’s Message to Clients Amid a Shifting Regulatory Environment: We’ll Continue To Support Our Clients So They Can Support Their Patients
Never has a change more significantly affected the clinical laboratory industry than that brought about by the FDA’s final rule regulating laboratory-developed tests (LDTs) as medical devices. In more than 500 highly detailed pages, the rule spells out daunting new requirements, a number of which are largely open to interpretation. Six months after the rule’s May 6, 2024, publication date, many labs that offer LDTs remain understandably overwhelmed and anxious.
The November 5 election of Donald Trump to a second term as president added yet another layer of uncertainty. Should labs proceed with preparations to comply with the rule knowing that a new Trump administration may initiate the process to rescind it? And what happens if a pending federal lawsuit challenging the FDA’s authority to regulate LDTs is successful?
ARUP Laboratories is asking the same questions in a fast-changing regulatory environment. For now, though, the rule remains in effect, with a May 6, 2025, deadline to comply with Stage 1 requirements looming. No one can predict when or how the new administration and Congress may act or what the court will conclude, so it is still prudent to prepare for Stage 1 requirements while closely monitoring how the landscape evolves, said Jonathan Genzen, MD, PhD, MBA. Genzen is ARUP’s chief medical officer, senior director of governmental affairs, and a leader of ARUP’s response to the rule, along with Julio Delgado, MD, MS, ARUP executive vice president, Adam Barker, PhD, ARUP chief operations officer, and Kristi Smock, MD, vice president of the ARUP Institute for Clinical and Experimental Pathology® (Research and Development).
Regardless of what happens, ARUP’s message to both clients and employees is unwavering: “Our eye will be on doing what we do best, which is supporting our clients, whatever happens, so they can best support their patients,” Chief Business Development Officer Julie Altwies said.
For more than a decade, ARUP has closely followed and actively engaged in the lead-up to the new rule and in every other effort to change the way clinical laboratories are regulated, Genzen said.
In the above video, ARUP leaders, including Adam Barker, PhD, Julio Delgado, MD, MS, Jonathan Genzen, MD, PhD, MBA, and Julie Altwies, discuss the new FDA rule and ARUP’s response.
Quality testing is and always has been paramount at ARUP, and each review of operations prompted by the specter of regulatory change produces the same result, Barker said. “We know exactly how to make safe, effective tests that provide accurate answers for patients. None of that has changed.”
“We’re very proud of what we’re doing and where we’re at,” he said. “We already have an exceptionally high-quality lab run by professionals who have dedicated their lives to the practice of laboratory medicine.”
He joined Delgado and Genzen in urging caution against overreaction as the clinical lab industry navigates uncertainty surrounding the rule.
By remaining flexible and staying informed, hospitals and health systems can be more thoughtful and strategic in how they’re going to adapt in a shifting regulatory environment while continuing to prioritize patient care, Genzen said.
Educating, Advocating on Labs’ Behalf

Key to ARUP’s partnership promise to its clients is its commitment to remain at the forefront of education related to the new FDA rule and any proposed changes to regulations affecting clinical labs. As an academic reference laboratory and a nonprofit enterprise of the University of Utah’s Spencer Fox Eccles School of Medicine and its Department of Pathology, ARUP shares knowledge and information as an essential part of its mission.
ARUP’s expertise on lab regulation manifests in webinars, LabMind podcast episodes, articles, professional society presentations, and numerous other resources that its medical directors have created and made available to help build understanding. (Refer to sidebar.)
Advocacy efforts that ARUP continues to lead are also important, Genzen said. “We want to make sure that the needs of all clinical laboratories, not just our customers, are reflected in any regulations that exist, be they part of the FDA final rule, or maybe legislative efforts going forward,” he said. “Being engaged is incredibly important right now for all clinical laboratorians and anyone who cares about this issue so that regulators, legislators, and the FDA better understand unintended impacts of their proposals.”
Sustaining a Robust Menu Amid Change

Critical to any discussion of the impact of the FDA rule and any future regulation is a reminder that ARUP’s expansive test menu remains intact. ARUP offers hundreds of LDTs, and any that were on the market before May 6, 2024, are exempt in their current form from FDA premarket submission requirements.
Even before the rule was published, ARUP had begun evaluating new tests in its pipeline and its planned updates to existing LDTs to decide how best to prioritize tests under the new regulatory framework that the FDA plans to phase in over five years. ARUP already submits tests through the New York Clinical Laboratory Evaluation Program (NY CLEP), one pathway allowed under the FDA rule. ARUP knows the NY CLEP pathway well and has developed strong relationships with New York regulators as a longtime NY CLEP-approved lab, Barker said.
He, Delgado, Genzen, and Smock oversee the prioritization of submissions for NY CLEP approval. “We prioritize tests by what’s best for the patient first, followed by what’s best for lab operations and efficiency, and only then by whether we see a market opportunity,” Delgado said.
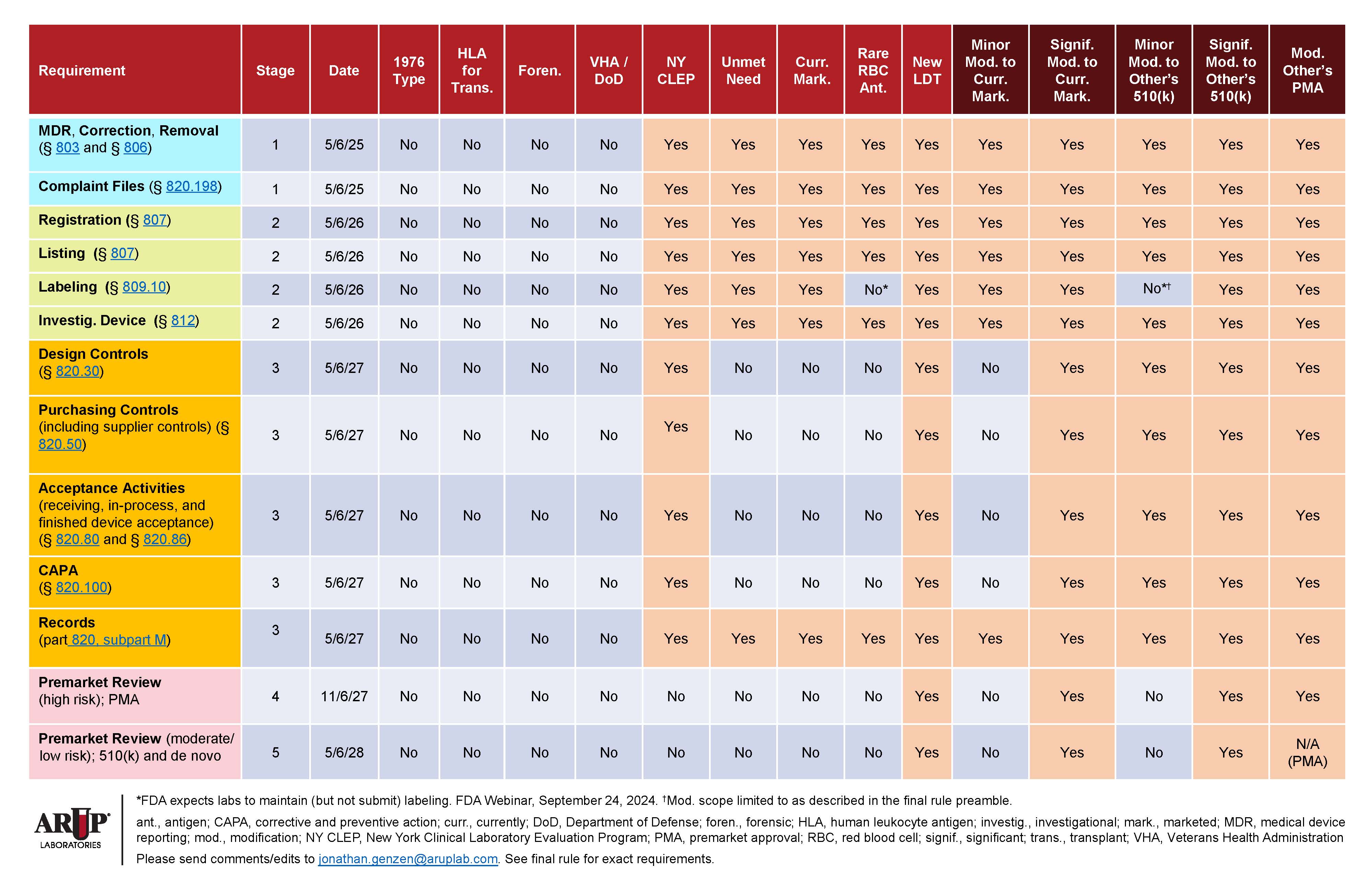
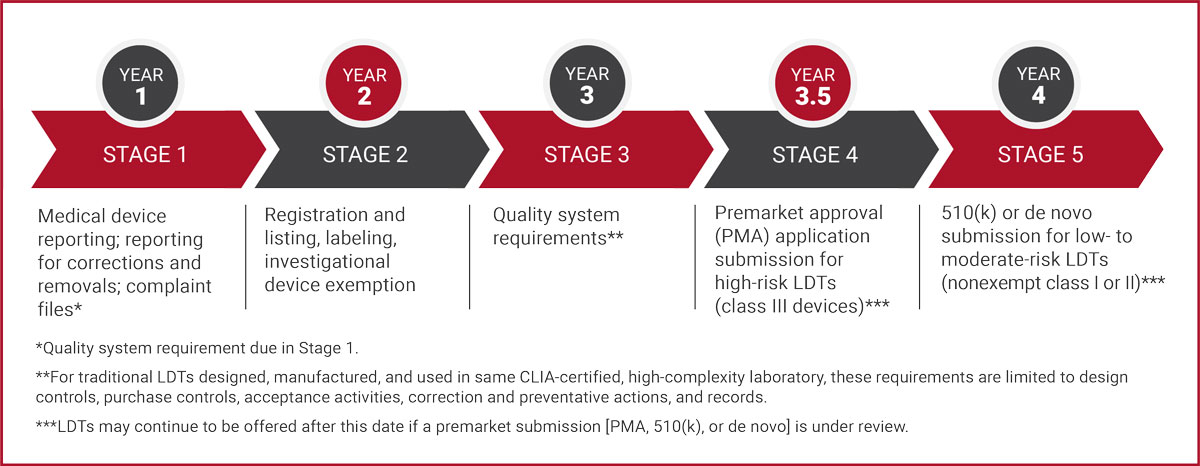
Simultaneously, work is well underway to understand and enable compliance with Stage 1 reporting required in year 1 and Stage 2 labeling required in year 2, as well as all future requirements if necessary. (Refer to Requirements Matrix and Timeline for Implementation.)
In every action related to the new rule or any other regulation, “We are being thoughtful, meticulous, careful, and also strategic,” Genzen said. “We will sustain our robust menu while also continuing to dedicate resources toward innovation and new test development. That’s incredibly important.”
Shifting Regulatory Environment
Following the presidential election, industry groups representing clinical labs acted quickly to urge the new Trump administration to rescind the FDA rule.
In a November 12 letter, American Society for Clinical Pathology (ASCP) President Greg Sossaman, MD, MASCP, urged members of the Trump transition team to act. The letter reminded transition team cochairs Linda McMahon and Howard Lutnick that the previous Trump administration prevented the FDA from regulating LDTs. In 2020, then U.S. Health and Human Services Secretary Alex Azar blocked FDA regulation of LDTs, basing his decision on a legal opinion drafted by the general counsel of the Department of Health and Human Services (DHHS) that the FDA lacked statutory authority to regulate LDTs.
ARUP has many ASCP members among its workforce, and ARUP is a laboratory member of the American Clinical Laboratory Association (ACLA), which has also been in contact with the Trump transition team, asking that President-elect Trump move to delay implementation of the rule and signal an intention to rescind it, a process that would take time.
Rescission of the rule would require that the FDA follow its established notice-and-comment rulemaking process, which, even if initiated early in President-elect Trump’s term, would take several months or longer.
Meanwhile, the lawsuit continues to wend its way through the court. The challenge started as two lawsuits, one filed by ACLA and the other filed by the Association for Molecular Pathology (AMP). They were consolidated in September in the U.S. District Court for the Eastern District of Texas. The lawsuits’ challenge is based on the belief that the FDA lacks legal authority to regulate LDTs as medical devices under the Federal Food, Drug, and Cosmetic Act, said Jonathan Carr, JD, ARUP’s chief compliance officer.
In June, the U.S. Supreme Court handed down a decision in the landmark Loper Bright Enterprises v. Raimondo case that is generally viewed as favorable to ACLA and AMP in their challenge to the FDA because the decision overturned the so-called Chevron doctrine. That 40-year-old legal precedent previously instructed courts to defer to federal agencies’ authority in reasonable interpretations of ambiguous laws.
The FDA has until December 23 to file its brief in response to the plaintiffs’ motion for summary judgment in the case. The judge could request a hearing on the motion in early 2025, and a decision on the motion would follow.
If the lawsuit is successful and the FDA under the new administration opts not to appeal, the rule will be terminated.
Even if the rule is rescinded or a court ruling leads to its demise, however, legislative attempts to further regulate labs may eventually follow, Genzen said.
The latest version of the Verifying Accurate Leading-edge IVCT Development (VALID) Act, which aims to add new regulation for LDTs, was reintroduced in the U.S. House of Representatives earlier this year, although Genzen said the future of the bill first introduced in 2020 will not be clear until after the new Congress begins.
Genzen said ARUP ultimately favors a solution that could result from collaboration between clinical labs, their trade associations, legislators, and regulators, all united in an effort to update existing quality standards for laboratories under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) while also doing what’s best for patients and what’s feasible for clinical labs.
He favors a compromise that would add transparency to LDTs currently offered by labs and keep beneficial aspects of test oversight under the CLIA framework while also potentially giving the FDA oversight for select high-risk tests, but not by simply lumping them in with conventional medical devices.
“There are some core principles that easily could be adapted for any future legislative or regulatory efforts, but it would require agencies that are willing to work collaboratively with the clinical laboratory community because it is in the interest of patient care,” Genzen said. “It shouldn’t be hard, and I’m still optimistic we can get there.”
For now, ARUP will continue to act as a resource and as an advocate for its hospital and health system clients and for the entire clinical laboratory industry, he said.
“There are more than 100,000 clinical laboratorians in the U.S. who go to work every day to conduct testing to take care of people in their moments of greatest need. This is their career effort, their contribution. What drives them to work is helping people,” Genzen said. “We obviously care about quality. We care about patient care. We care about transparency.
“We’re committed to finding the right solution. I think there is a right solution if we all work together.”