James Hadley, CISSP, chief information security officer, brings 25 years of cybersecurity experience to ARUP with a focus on risk management, collaboration, and building a resilient security culture.
In his new role, Adam Barker, PhD, will lead operational innovation to support ARUP’s growth while maintaining the highest quality.
BRIDGEWATER, N.J.—Recordati Rare Diseases Inc.
Tracy George, MD, ARUP chief scientific officer and Innovation Business Unit president, will help advance life sciences innovation and strengthen Utah’s healthcare ecosystem with BioUtah.
ARUP experts will showcase their latest research at the ASH Annual Meeting and Exposition, including updates on key clinical trials and the development of automated disease scoring.
Paul’s life was saved thanks to 12 generous blood donors, demonstrating the power of community and compassion.
ARUP Advocates for the RESULTS Act To Protect Patient Access to Critical Laboratory Testing Services
The RESULTS Act offers meaningful reform to a flawed system and will ultimately benefit patients, support innovation, and create stability within the clinical laboratory community.
Jonathan Genzen, MD, PhD, MBA, ARUP chief medical officer and senior director of governmental affairs, is taking the mic of the LabMind podcast and continuing its legacy of insightful conversations.
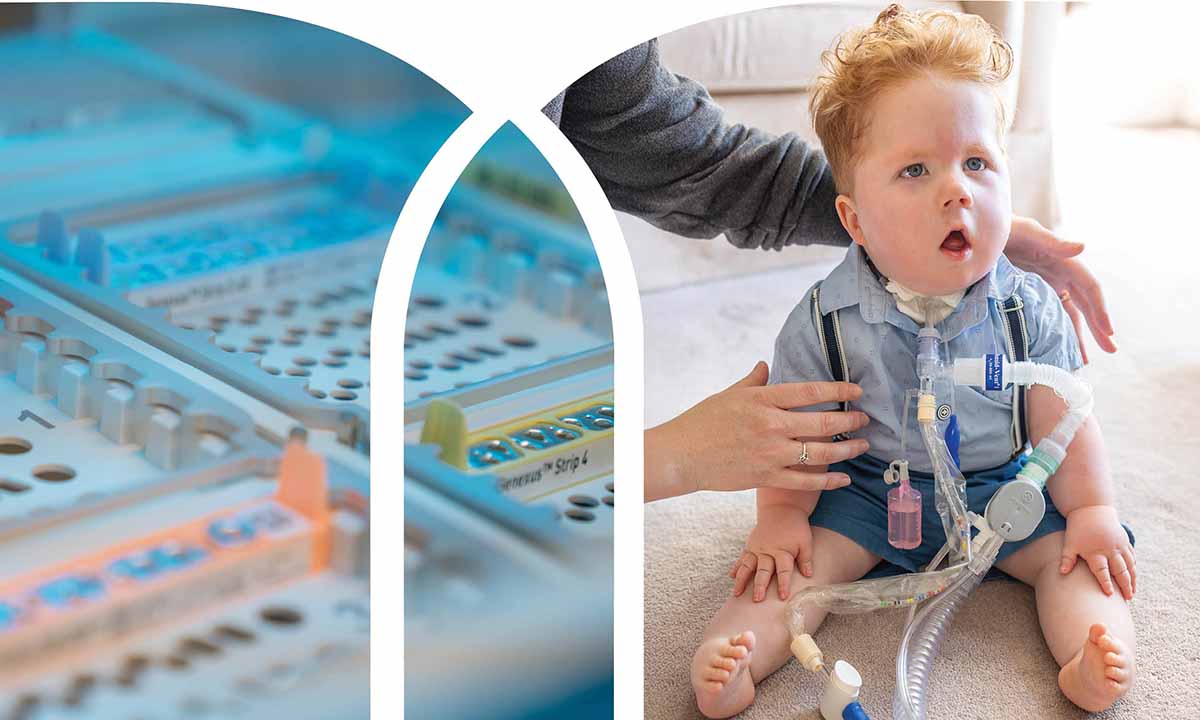
The Utah NeoSeq Project, a collaboration between ARUP Laboratories and University of Utah Health, is transforming neonatal care using rapid whole genome sequencing (rWGS).
ARUP has used novel statistical modeling approaches to establish age-specific reference ranges for pediatric patients, facilitating more accurate diagnoses of G6PD deficiency.
The FDA and the National Comprehensive Cancer Network (NCCN) urge DPYD testing before chemotherapy. ARUP offers guidance and resources to help clinicians choose and interpret genotyping tests.
ARUP experts will highlight the latest in genomic sequencing at the upcoming National Society of Genetic Counselors (NSGC) Annual Conference.