Tapping the Lifeblood of Marrow for Transplant Patients
Whenever Lauren Christensen gets a Frosty at Wendy’s, she thinks of one of her patients. “After each bone marrow extraction, she always treats herself to a Frosty,” says Christensen, an ARUP technician who specializes in bone marrow extraction and biopsies at Huntsman Cancer Hospital for patients with blood cancers.
“I’ve known some of these patients for years; it’s why I love my job. You get to know about their kids, pets, how school is going,” says Christensen, who hopes the conversation helps distract patients from the procedure.
The extracted bone marrow or a bone biopsy helps pathologists identify the type of cancer (e.g., Hodgkin/non-Hodgkin lymphoma, leukemia, myeloma). Follow-up extractions allow for monitoring to see if the treatment is working. Treatment for these patients usually involves chemotherapy or radiation. If this is not working, then a bone marrow transplant (BMT) may follow, which aims to replace unhealthy blood-forming cells with healthy ones.
Christensen assists clinicians, guiding them to ensure that what they extract is bone marrow and that there is enough of it for sufficient testing—usually about two teaspoons. Marrow is the soft tissue inside bones that produces more than 200 billion new blood-forming cells daily—cells that will grow into red blood cells (oxygen carriers), white blood cells (infection fighters), and platelets (clot and repair). Marrow is the seedbed of our blood.
A deep red, with a viscosity that is thicker than blood, marrow is filled with tiny bone spicules that look like grains of sand. Before it clots, Christensen will immediately, while still in the room, plop marrow droplets onto slides. Then blood from a quick finger prick is put on a slide as well. This allows the pathologist to compare the circulating blood to the bone marrow slides to see how the two compare.
In the lab, these slides will be analyzed by a pathologist such as Mohamed Salama, MD, ARUP’s chief of Hematopathology. “When I look at a patient’s blood sample through the microscope, I determine the tests we are going to need to do from that point forward to get more specific info—with each test, results may indicate a need for another test, until we’ve narrowed the differential diagnosis down to a very specific diagnosis.”
After pathologists identify the type of disease, then they look for clues to indicate risk factors for that particular patient, such as genetic variations, blood cell count, plasma counts, and chromosome variation. All these results play into a scoring system that will guide the doctor to move forward with a transplant or choose another treatment approach.
After pathologists identify the type of disease (e.g., type of leukemia), then they look for clues to indicate risk factors for that particular patient, such as genetic variations, blood cell count, plasma counts, and chromosome variation. All these results play into a scoring system that will guide the managing doctor on whether to move forward with a transplant or choose another treatment approach. “We provide the doctor with a very detailed story of what is going on based on all these clues,” adds Salama.
After pathologists identify the type of disease (e.g., type of leukemia), then they look for clues to indicate risk factors for that particular patient, such as genetic variations, blood cell count, plasma counts, and chromosome variation. All these results play into a scoring system that will guide the managing doctor on whether to move forward with a transplant or choose another treatment approach. “We provide the doctor with a very detailed story of what is going on based on all these clues,” adds Salama.
The Heart and Science of Matching
There are two different types of bone marrow transplants: one involves transplanting the patient’s own cells back into the body; this is an autologous transplant. These blood stem cells are taken from the blood before chemotherapy and then transfused back in after the treatment. Transplants that use another person’s cells are known as allogeneic transplants and require finding the right match.
The search to find the right match can be quite extensive, generally starting with siblings and parents, then extended relatives, and then eventually reaching out to nonrelated people or donor registries. This process can take on average about three months (to day of transplant) and can be longer for minorities and mixed ethnicities, who may be more limited in finding a match because the potential matching donor population pool is smaller.
Each sibling who has the same biological parents has a 25 percent (1 in 4) chance of being a match. However, 70 percent of patients will not have a match in their family. In 2016, there were about 14,000 people ranging from infants to elders looking for a match in the United States.
What needs to match up are the human leukocyte antigens (HLA) between a donor and recipient, which are specific proteins on the surface of white blood cells and other cells that make each person’s tissue type unique. Blood tests can show if a person’s HLA is a good match for a patient—the better matched, the less likelihood of complications.

“These HLA molecules are inherited from your parents, one set from each [known as a haplotype]. If you have siblings, there is a 25 percent chance that you will have an HLA identical sibling—the best match for a transplant—and 25 percent chance that you share none at all, in which case a transplant is not possible,” explains Eszter Lazar-Molnar, PhD, ARUP’s medical director of Immunology. “These molecules show a strong linkage to racial and ethnic background.”
For those who can’t find a match within their family, it is crucial that bone marrow registries, like the National Marrow Donor Program, are available and have a large pool of donors. “By registering to be a donor, you can literally save someone’s life by donating once,” says Lazar-Molnar who also oversees the University of Utah’s Histocompatibility and Immunogenetics Laboratory. Her team re-confirms that the blood typing is accurate for those who find a match through registries.
Lazar-Molnar explains that while a perfect HLA match is best, sometimes this is not possible, and patients—involving parents and children—must settle for mismatches in which only some of the HLA molecules are matched but not all of them, which may increase the risk of rejection and other complications. There is a 50 percent chance of sharing one haplotype with your siblings, and with each of your parents—therefore the chance of finding a donor with one haplotype match is usually much easier. Haplo-transplants raise other issues: mainly, how will recipients react to the mismatched HLA molecules being introduced into their body via the donors’ bone marrow or stem cells? In short, can everyone play well in the sandbox together or not?
Laboratory tests can be used to detect if the recipient has antibodies that will react to the donor’s HLA and help monitor and manage these interactions. Such antibodies could potentially interfere with the body accepting the new cells, and they may end up injuring the donor cells by triggering an immune response. “Not everyone has antibodies that strike against HLA, but previous transfusions, transplants, or pregnancy may lead to sensitization and the development of HLA antibodies,” says Lazar-Molnar.
Donors can now donate either through peripheral blood draws, or by having a bone marrow extraction. For collection of stem cells from peripheral blood, donors take medication that increases the number of blood-forming cells in the blood stream, which then can be collected by passing the blood through a machine that separates them. Transplant involves injecting these cells into the recipient’s body, and the cells find their niche—migrating to the recipient’s bone marrow—and go to work generating healthy blood cells.
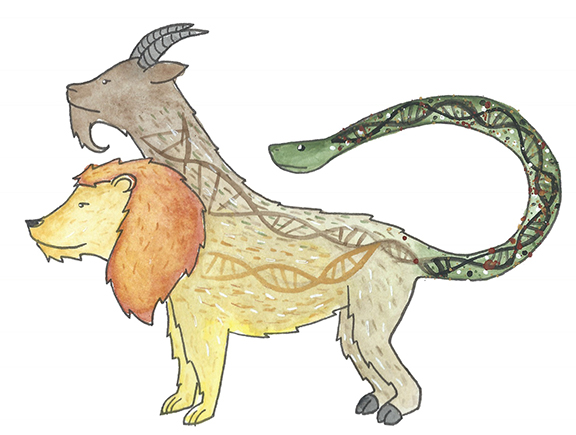
The patient becomes a chimera, with DNA from another being.
“The match testing is happening while the doctor is preparing the patient for a transplant—eradicating the disease or decreasing the bulk of it through chemotherapy or other disease protocols,” explains Salama. Pathologists are an integral part of the medical team to help identify when a patient is at the best point for a transplant to begin.
To Reject or Accept: A Delicate Balance
Right after a transplant, the doctor will check blood counts weekly to see if new blood cells are starting to grow in the bone marrow. Pathologists are able to identify which cells are from the donor (by markers in the DNA) and which ones are the recipients in the patient’s body in order to determine if the transplant (graft) is working or if there is any impending rejection or recurrence .The patient becomes a chimera with DNA from another being. A chimera is the Greek mythological beast that consisted of parts from different animals.
If there are more of the donor’s blood cells (DNA), this indicates success and less possibility of graft rejection. If there are more of the recipient’s blood cells, then this may indicate a relapse or rejection. A very serious concern is complication called graft-versus-host disease (GVHD), in which the donor’s cells identify recipient’s cells as foreign and start attacking not just the recipient’s unhealthy bone marrow cells, which is good, but also start attacking other parts of the body.
“Imagine an army of praying mantises swarming a rose bush and eating away the aphids that are killing the roses, but after depleting the aphids, they turn on the roses themselves,” says Nahla Heikal, MD, who analyzes post-transplant chimerism blood testing. She is a medical director of Immunology and Hemostasis/Thrombosis at ARUP.
She must closely monitor patients to make sure their immune systems are suppressed enough to accept the proliferation of donor T-cells, but not suppressed so much that GVHD develops. “It’s a very fine line to keep in control,” admits Heikal. T-cells, a type of white blood cell, are fundamental to our immune system; they are like soldiers that search out and destroy the targeted invaders (pathogens).

If the patient’s health is progressing, then the blood tests become less frequent. If there is still concern, then testing will continue, sometimes for many months or years. Testing can reveal how certain cell subsets are doing (e.g., myeloid cells, T-cells, B-cells, natural killer cells). "These can provide us with different messages,” explains Heikal, “for example, if there is a high percentage of donor T-cells on day 14 after the transplant, then this reassures us that things are going well.” Looking at these subsets can also provide early warning signs of an impending rejection or recurrence alerting the clinician to early treatment.
“As I do calculations to understand and interpret the results, I’m very conscious of the person who is at the other end of these tests,” expresses Heikal. She knows doctors will be looking at the results as a progression—or not—to determine if the patient is doing better. “I know how meaningful these numbers are for determining the best treatment and what they will mean for that patient.”
The Gift of Life, Twice: Cord Blood Donation
When a baby enters the world, the gift of life can be magnified if the umbilical cord blood is donated to a public cord blood bank. Cord blood an be used to treat more than 80 diseases, including blood cancers like leukemia and lymphoma. The need is intense. According to the National Marrow Donor Program (NMDP), every three minutes, someone is diagnosed with blood cancer in the United States.

Studies show that cord blood does not need to match as closely as bone marrow or peripheral (circulating)blood for a successful transplant. For transplant patients who don’t have a matched donor in their family, this increases the likelihood of finding a match—a process that can take more time than a patient might have.
Typically, the umbilical cord and placenta are discarded after a baby is born. Some people make arrangements to have their child’s stem cells—from the cord and placenta—collected and stored in a private cord blood bank in case of health issues later in the baby’s life or to secure the cells for a biological sibling who has a diagnosed medical need. To be clear, these stem cells are not taken from an embryo, and no blood is taken from the baby.
Sometimes finding a match is more difficult for those of different ethnic backgrounds (including interracial) because they have a smaller pool to draw matches from than Caucasian (white) donor seekers. In 2015, about 10 times more African-American patients could not find a match compared to 3 percent of Caucasians, according to NMDP, which operates Be The Match, the world’s largest and most diverse donor registry. Donating cord blood increases the likelihood that a match can be found.
While only certain U.S. hospitals collect cord blood for donation to public cord banks, NMDP can send a cord blood donation kit to anyone who requests one.Donating is free and safe for both the baby and mother.
It’s a profound beginning when a newborn is able to gift another human being a second chance at life.

















 HOME
HOME