From Your Sister with Love— the Gift of Life 
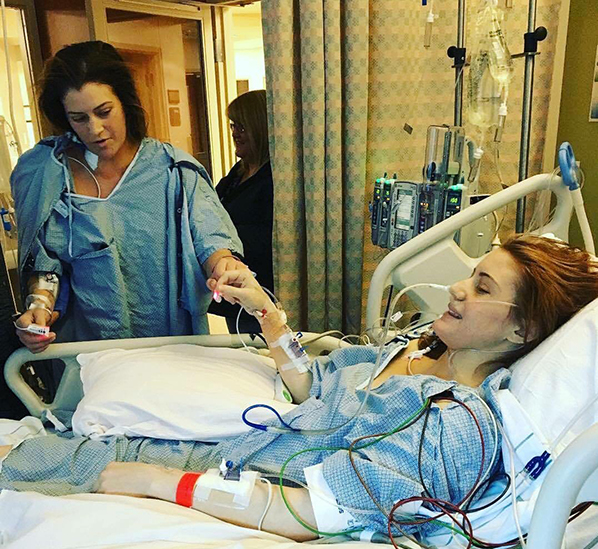
Two sisters give each other a celebratory fist bump after liver transplant surgeries. Chelsie (standing) donated 60 percent of her own liver to Kylie. They are the second liver-transplant pair to participate in the University of Utah Health’s Living Donor Program.
When Kylie Sharp slides open her closet door, a blush of bridesmaid dresses lines the far side. A navy- blue one hangs among the pink ones—all silky remnants of unattended or barely made weddings. As Kylie’s close college friends walked down aisles to exchange vows, her life was riddled with doctors’ appointments and hospital stays. “It’s not a good thing when everyone in the ER knows your name,” quips Kylie.
Nine years ago, Kylie was diagnosed with autoimmune hepatitis. She was 17 years old. At first, her family thought the fatigue was from her busy high school schedule. A competitive gymnast, Kylie was training five hours a day, five days a week. Then the jaundice kicked in and she learned of her diagnosis. Her doctor informed her that she would eventually need a new liver. Kylie was added to the national waiting list, in which the sickest patients are given priority, but she also had a backup. “When I first heard the news, I just knew that if I was a match, I would donate part of my liver,” says Chelsie, Kylie’s older sister by two and half years.
Waiting to live is not part of Kylie’s DNA, as is evident in her blog, “LiverDie.” This self-described “shy” young woman (“except in the gym”), with striking long, auburn hair and a wide-open smile, set off for Seattle, where she was attending the University of Washington on a full gymnastic scholarship.
In her freshman year, a physical required of all school athletes revealed that Kylie had developed primary sclerosing cholangitis, a chronic condition that damages the bile ducts and eventually compromises the health of the liver. This reaffirmed the need for a new liver.
“She keeps me calm, and I make sure she stays brave.” Chelsie Sharp
Kylie fought through her fatigue and any thoughts of quitting, continuing with her rigorous training schedule. “I was always tired, but I just dealt with it because that is always how I felt.” While earning a degree in anthropology, Kylie headed to the Bahamas on a school service trip and studied abroad in Tahiti.
She settled in Seattle after college, but every few months she would get sick and end up returning home for care. Then University of Utah Health announced its new Living Donor Program for liver transplants; Kylie qualified. She called her older sister, Chelsie, and gave her the update—she was moving home and gearing up for a new liver. Chelsie was ready. Her sentiments had not changed.
“Hello beautiful human…The last eight years of my life has lead me to a profound knowing that life is a precious gift…How we spend our time is how we spend our life, so wake up and LIVE. Live every day, live every moment, live through every smile and live through the tears….We are never sure where this journey leads or where it ends.” *
“It just naturally felt like my role—it was like a reflex,” says Chelsie. They would be the second liver-transplant pair to participate in the Living Donor Program. For the past decade, the program had focused only on kidney transplants—about seven times more patients are waiting for kidneys than livers (UNOS).
“This program meant I wouldn’t have to get even sicker before I became eligible for a transplant. It’s better if you can be stronger when you go through it,” says Kylie.
The Amazing, Regenerating Liver
Shaped like a partially deflated football, the liver is the largest internal organ, weighing two to three pounds in adults. It is vital. We can’t live without it. The liver helps us process what we eat and drink into energy and nutrients for our bodies. It is the main detoxifying organ in the body, removing harmful substances from our blood.
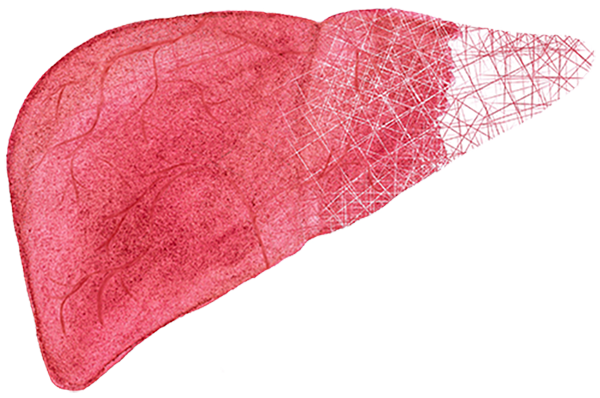
Unlike any other internal organ, the liver can substantially regenerate and grow to just the right size for the body it is in—as little as 25 percent of the original liver mass can regenerate back.
The prospect of liver regeneration was introduced in the early 19th century, but the concept is captured in the story of Prometheus, the Greek god whose immortal liver was feasted on day after day by Zeus's eagle, his liver miraculously regenerating each night. Of course, this is more myth than medical literature.
The regeneration process begins immediately after surgery. By three months, the donor’s liver will have grown to 90 percent of its original size. The recipient’s liver typically grows more slowly but will grow to the size required for normal liver function.
Before surgery, 3-D imaging is used to calculate the size and volume of the donor’s liver and guide surgeons on how much to take. “We know how much will be left for the donor and the actual volume that is being given to the recipient. This ensures that both will have sufficient liver to function if growth was not to happen—which is highly unlikely,” says Robin Kim, MD, surgical director of liver transplantation, and chief of the Division of Transplantation and Advanced Hepatobiliary Surgery at the University of Utah. While a person can live with as little as 25 percent of her liver, Kim emphasizes that their patients have far more than that.
The option of securing an organ from a living donor helps bypass the national waiting list for an organ. According to the Organ Procurement and Transplantation Network, every 10 minutes, someone is added to this list and an average of 22 people die each day waiting for a transplant. Even though one organ donor can save eight lives, the need far outpaces the demand. This is why every donor—whose body harbors multiple organs—is so precious.
Match Making
Before Chelsie could donate part of her liver to her sister, both were scrutinized in different ways. Was Chelsie truly ready and committed to this decision? Was her own liver healthy enough? Were Chelsie’s and Kylie’s bodies free of infections and viruses?
“The rigor they put you through to ensure you are up for being a donor is intense. You go through months of seeing if you are a good fit,” says Chelsie. A team of people were involved, including doctors, social workers, a psychiatrist, and a dietician, among others. Questions ran the gamut: How does your family feel about this? Is your workplace supportive? Financially, how will you be affected? Whose idea was this? “It was like a series of tough job interviews,” recalls Chelsie, who didn’t budge in her decision. “It had been made.”
While the transplant process involves a multidisciplinary team all working closely together, pathologists are involved in the entire arc of care, from pre-transplant to post-transplant. Sometimes, they monitor patients for years after a transplant. More often found in the lab than at the patient’s bedside, the pathologist’s expertise is in analyzing a patient’s blood and tissue biopsies on slides beneath a microscope.
Pathologists determine blood types, antibody status, and how well an organ is functioning. They are able to check for infections and the presence of autoimmune or inherited diseases in both the donor and recipient. “These diagnostics help us determine if transplantation is a good idea or not,” says Kim.
While the transplant process involves a multidisciplinary team all working closely together, pathologists are involved in the entire arc of care, from pre-transplant to post-transplant. Sometimes, they monitor patients for years after a transplant.
To avoid transplant rejection, Kylie’s and Chelsie’s blood is carefully studied, as well as their liver tissue, which needs to match as closely as possible. If Kylie’s body detects something foreign—antigens from Chelsie’s liver—her body might attack her new liver. (Antigens are perceived as a foreign substance in the body and trigger an immune response).
Sometimes the donor’s history is unknown or incomplete, especially in the case of donors who have passed away. The liver is screened for inherent liver disease, hepatitis, scarring of the liver, fatty infiltration, liver spots, or anything else that might indicate disease. “The pathologist will help us determine what the disease is and how far along it is,” says Kim, who notes that lab results are just one of the guiding factors used to determine whether a transplant is a good idea. “It’s a go about 50 percent of the time.”
“In the pre-transplant stage, we’re looking for what infections the donor and the recipient may have been exposed to in the past,” explains Kim Hanson, MD, MHS, section chief of Clinical Microbiology at ARUP. She is also at the bedside, caring for patients. Hanson explains that many of us have been exposed to and harbor asymptomatic infections in our bodies, such as the herpes viruses or tuberculosis. “These can wake up after a transplant and cause major problems for the recipient. Lab testing is done to screen both the donor and the recipient. “We then use the results to develop infection prevention and/or monitoring strategies for the recipient,” says Hanson. The risk of infection varies based on the type of transplant.
Sometimes, mid-surgery, the surgical team will come across something suspicious (e.g., a mass, an enlarged lymph node), and they will biopsy it and wait for a pathologist to analyze the tissue. “They need to know more about it before they proceed. Is it cancerous or not?” says Allie Grossman, MD, PhD, medical director of Surgical Pathology and Molecular Oncology at ARUP. She and her colleagues may have only 20 minutes to receive the patient’s tissue, mount it, section it, stain it, and interpret it in order to provide a diagnosis. If the tissue is cancerous, the transplant is halted.
If this complex matchmaking process goes well, and the donor’s organ proves healthy, then the next milestone is the actual transplant.
When I Woke Up
Seven years after Kylie learned she would eventually need a new liver, she and her sister were being prepped for surgery in the U of U’s surgical transplant unit. It was 7 a.m., and their family was gathered around Chelsie’s hospital bed.
“I remember everyone being nervous,” says Kylie. A nurse was inserting an IV tube into Chelsie’s arm, and she had to be poked twice. Chelsie hates needles; she passed out on her very first blood draw during the matching process. “You sure you want to do this?” teased Kylie. “When you wake up, there are going to be a lot more of these around you.”

Four hours later, it was Kylie’s turn to leave her mom’s side. “I love you,” said her mom, Toynet Sharp. “You’re going to feel really good when you wake up, and I’ll be here waiting for you.” She wouldn’t see Kylie until 10 p.m. Chelsie woke up at 3 p.m.
“It was hard to sit there and wonder,” recalls Toynet. “What kept us going was the steady stream of updates we got on each of the girls throughout the surgeries. It was really reassuring.” Coincidently, it was the same day as her and her husband’s 30th anniversary. It was not the “extra special thing” they had planned on, but when they found out the date, it seemed like a good fit. “One daughter helping save the life of our other daughter was about as extra special as it gets.”
Kylie recalls: “When I woke up, I was crying I was so happy to see my mom. How’s Chelsie? Is she OK?” Kylie wanted to know. She could see Chelsie through the door in the adjoining ICU room. Kylie attempted three steps to go see her sister, but had to lay back down.
“When I woke up, my first concern was my mom—her whole world is her three children,” remembers Chelsie. “And two of us were in surgery.” Once Chelsie was able to stand with the care team’s help, she shuffled over to her sister. With Chelsie leaning on Kylie’s bed, the two sisters with two groggy smiles gave each other a wobbly we-did-it fist bump.
A team of 10 doctors and nurses transplanted 60 percent of Chelsie’s liver into Kylie, completely removing Kylie’s weakened liver. Because the liver is a highly vascular organ, the process is slow and meticulous work, about a four- to five-hour operation for each. Within a few days of surgery, Kylie’s jaundice began to disappear, the clotting abilities of her blood improved, and she began thinking more clearly. “My body just felt better,” says Kylie.
Now, the next milestone: Kylie’s body needed to accept the new liver.
“Be Alive While You Can”
Kylie began receiving high doses of immunosuppressant drugs just hours before the transplant. Immunosuppressant drugs cast a sleepy spell over the cellular warriors in the body that fight foreign invaders, preventing them from attacking the new liver, mistaking it for an intruder.
However, when these warriors are subdued, they can’t fight off the real threats of viruses, germs, and bacteria. “It is a really delicate balance on how much to give,” admits Kim Evason, ARUP medical director of Anatomic Pathology. “If you don’t provide enough of the immunosuppressant drug, then you risk rejection; if too much, they may develop an infection.” Evason analyzes more than 500 slides a week looking for clues that will help guide doctors toward the best treatment for their patients. “We look at the slides and the patient’s chart and then start puzzling it together.”
“I tell my patients that transplantation is about second chances and that many don’t often get that chance at end-stage organ disease
Yes, there are going to be ups and downs, but at the end of this process is the opportunity to lead a normal and healthy life.”
Robin Kim, MD
Last August, only five months after her transplant, Kylie was diagnosed with post-transplant lymphoma cancer, an accelerated growth of white blood cells in the body’s immune system. “I thought, this time what can I give her?” says Chelsie. The Epstein-Barr virus (EBV) is often the underlying culprit that triggers this type of lymphoma; most likely Chelsie had been exposed to this virus and it woke up from a latent or dormant state once it was introduced into Kylie’s body. EBV-driven cancer is relatively rare, developing in approximately 1–2 percent of liver transplant patients within the first five years after a transplant. (To learn more about the pathophysiology, see sidebar).
While a team of medical specialists guided Kylie through her treatment, it required a deluge of strict doctors’ appointments, unexpected hospital stays, and days of feeling “ugh.” “She spent more days in the hospital than she did in her own bed last year,” recalls Chelsie.

It was the year many of her close friends were marrying, and Kylie never knew, until the last minute, whether she was going to make it or not. It depended on her health. Despite the frustrations, Kylie focused on the good days. “If Kylie can, she is totally out living life,” says Chelsie. “Her attitude is: Be alive while you can.”
“When I feel good, I want to go out and play and do what it is I love. I try not to think about what is holding me back,” says Kylie, who loves dogs, rock climbing, and hiking. “‘You were just in the hospital yesterday, what are you doing out hiking today?’ I get that question all the time.”
Maybe it was all that time spent balancing—walking, leaping, dancing—along the unforgivingly narrow path of a gymnast’s beam that has helped Kylie develop her grit for counterbalancing her trials with positivity—admittedly an emotionally thin line at times. It is an art, a discipline she is passing on to young gymnasts as a coach.
Now when Kylie slides open her closet door, a long, pink chiffon dress is mixed in with the other pink bridesmaid’s dresses. This past September, when Kylie watched her sister Chelsie walk down the aisle to exchange vows, she wore this dress, feeling healthy and happy for everyone.
“Without the doing, the dreaming is useless. We can wake up every morning and day dream about the life that we want to live or we can choose to make that life our reality. I don’t know about you, but I want my dreams to be my reality. So, here’s to getting out there and exploring every chance that I get and to create a life that I can’t wait to share.” *
*Excerpts are from Kylie Sharp’s blog, www.LiverDie.net. Her instagram account: liv_er_die.
Living Donor Programs
Last year, University of Utah Health launched its Living Donor Liver Transplant Program, one of only 20 such programs in the country. So far, five people have successfully received a living liver transplant; Kylie and Chelsie Sharp (see main article) were the second pair to participate in the program.
Living donor transplantation, in which a part (liver) or whole (kidney) organ is donated by a living person—often a family member or friend—increases the availability of healthy organs for transplants so that recipients can undergo a transplant before they become increasingly sick or die as a result of organ failure.
According to the American Transplant Foundation, in 2015, only 359 liver transplants (or about 4 percent of all liver transplants performed that year) were made possible by living donation.
Living donor kidney transplant programs are more common, with some 230 across the United States. The U of U Health’s own program started in 1966 and has provided 950 kidney transplants, with over a 98 percent patient survival rate.
“This disparity in numbers of living donor kidney versus liver programs is due to multiple factors, including the increased complexity of the surgery and the later inception of living donor liver transplantation,” says Robin Kim, MD, surgical director of liver transplantation and chief of the Division of Transplantation and Advanced Hepatobiliary Surgery at the University of Utah.
Living donor programs have a positive ripple effect, giving others on donor transplant waiting lists a better chance of becoming a recipient of a deceased donor. According to the American Liver Foundation, currently some 17,000 children and adults are waiting for donated livers. The waiting list grows every year.
How Can a Transplant Trigger Lymphoma Cancer?
The Epstein-Barr virus (EBV) is a common herpes-type virus that infects about 95 percent of adults. At the time of initial infection, EBV may lead to the disease called mononucleosis or “mono,” or it may cause no symptoms at all. Once we are infected with EBV, the virus remains with us for life. Alternatively, some patients may never have been exposed to EBV before a transplant, and it is possible to acquire the infection through a transplanted organ if the donor was previously infected.
EBV resides in blood cells called B lymphocytes in a resting or latent state. In the latent state, the virus changes the way its genes and proteins are expressed so that it can evade the immune system and remain in the B cells of our bodies undetected. The genes and proteins of latent EBV also stimulate B cells to proliferate or divide and make more copies. The virus then gets passed along in these cell divisions.
Some of the rapidly proliferating or dividing B cells may turn into cancer cells. When the immune system is normal and healthy, it keeps abnormal B-cell proliferation in check. When the immune system is weakened by medications after a transplant, uncontrolled B-cell proliferation can turn into cancer (e.g., lymphoma).
The main treatment for EBV-driven lymphoproliferative disease is a reduction in the immunosuppressive drugs. The hope is that this will allow the immune system to help fight the abnormal B-cell proliferation. If that does not work or the disease advances to cancer, then treatment entails chemotherapy.

















 HOME
HOME