A better estimate of menopausal status will help providers and patients address both the symptoms of menopause and the associated risks, such as cardiovascular disease and osteoporosis.
ARUP Laboratories now offers a clinically validated and FDA-cleared immunoassay, developed by Ansh Labs, to determine menopausal status. The test will aid providers in treating individuals who are experiencing menopausal transition and in managing the health risks associated with decreased estrogen production.
“Treatment options exist, and the more information that women have, the more proactive they can be about their future health,” said Joely Straseski, PhD, MS, MT(ASCP), DABCC, FADLM, section chief of Chemistry and medical director of Endocrinology at ARUP Laboratories. Straseski has been instrumental in this addition to ARUP’s test menu.
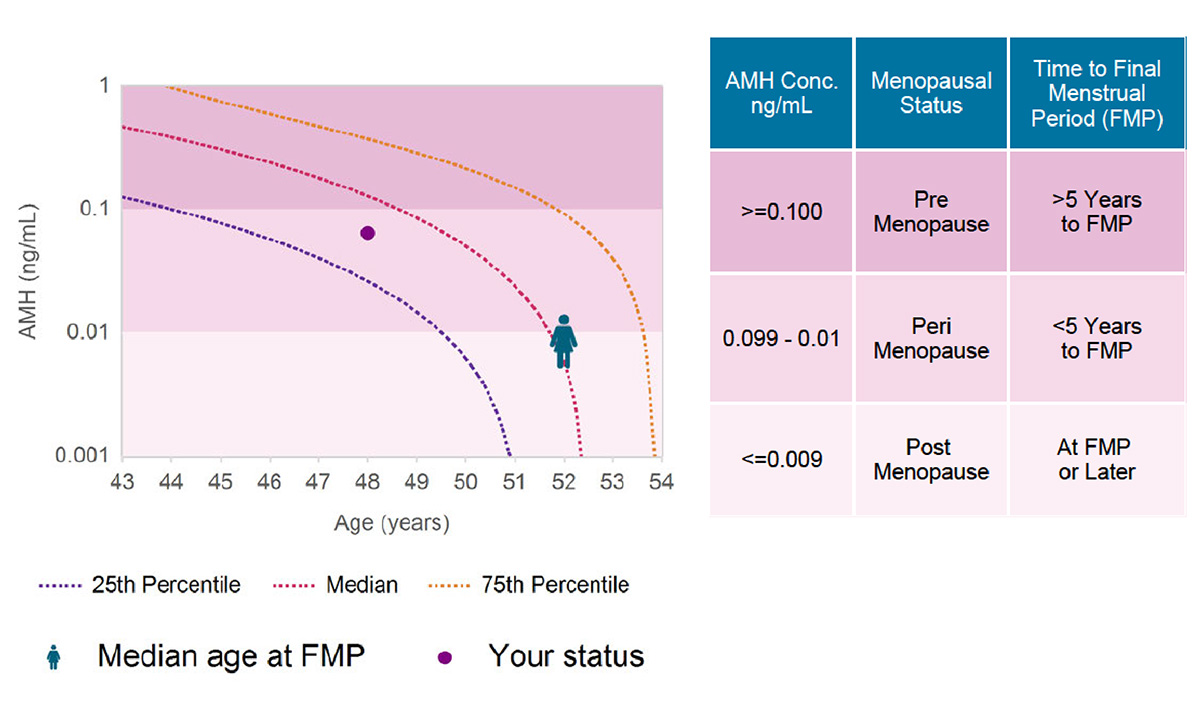
The menopausal status test is called MenoCheck, and it utilizes ultralow concentrations of anti-Müllerian hormone (AMH). AMH is an indicator of ovarian reserve and may be used to determine whether a woman is more or less than 5 years from a final menstrual period.
“As a hormone produced by the follicles, AMH is a more direct measurement of ovarian functionality, and it is not predicted to fluctuate much through the menstrual cycle. Therefore, it’s a more practical and reliable marker than those currently used to assess menopausal status,” Straseski said.
According to Camille Moreno, DO, MSCP, medical director of the University of Utah Obstetric and Gynecology Department’s Midlife Women’s Health and Menopausal Medicine program, individuals going through menopausal transition experience not only classic symptoms, such as hot flashes and night sweats, but they can also experience joint pain, brain fog, cognitive changes, mood swings, and worsening migraines.
“By the time I see patients in the clinic, they’ve been suffering for years,” Moreno said. “I think we have a different standard for women’s tolerance and suffering. They feel they should be able to survive this stage, even if their symptoms are truly severe and affecting their quality of life.”
Not only do women experience debilitating symptoms, but decreased estrogen production is associated with significant health risks, including cardiovascular disease, declining bone health, and even increased risk for Alzheimer’s disease and dementia.
“Estrogen is considered a protective hormone, and the loss of estrogen production is absolutely a risk to long-term health,” Straseski said.
According to Moreno, the onset and duration of perimenopause can vary widely among individuals. The uncertainty can make treatment decisions more difficult.
“Perimenopause occurs anywhere in your forties, and symptoms last anywhere between seven and 10 years, which is a ballpark estimate and not specific to each patient,” Moreno said. “Results from a blood test may help individualize that information and facilitate treatment decisions.”
The MenoCheck test uses AMH concentrations measured by the picoAMH test, which is capable of detecting very low AMH concentrations. The picoAMH test was developed by Ansh Labs, a developer and manufacturer of tools for biomedical research and clinical diagnostics. The company focuses on women’s health, reproductive endocrinology, obstetrics/gynecology, and complications associated with fertility and infertility, according to Tom Verghese, president of Ansh Labs.
"We recognized that many women struggle to navigate the confusing symptoms of menopause, often hampered by a lack of reliable diagnostic tools for determination of their hormonal status,” Verghese said. “The exceptional accuracy and unparalleled sensitivity of picoAMH now offer women and their healthcare providers a powerful tool to understand, manage, and optimize their menopausal transition care through the MenoCheck test.”
The MenoCheck test received FDA clearance in October 2018 for the determination of menopausal status in women starting at 42 years of age. The test is based on a subset of data from the Study of Women’s Health Across the Nation (SWAN), a longitudinal study designed to improve understanding of the experiences of women during midlife and aging. The study began in 1994 and has collected data from 3,302 eligible participants for approximately two decades.
Moreno encourages both providers and patients to proactively address menopausal transition.
“There’s really no need for suffering throughout this stage of life. We have new data and new evidence that support women receiving the treatment options available, whether it’s hormonal or nonhormonal. No one should be suffering alone anymore,” Moreno said.
Kellie Carrigan, kellie.carrigan@aruplab.com
















