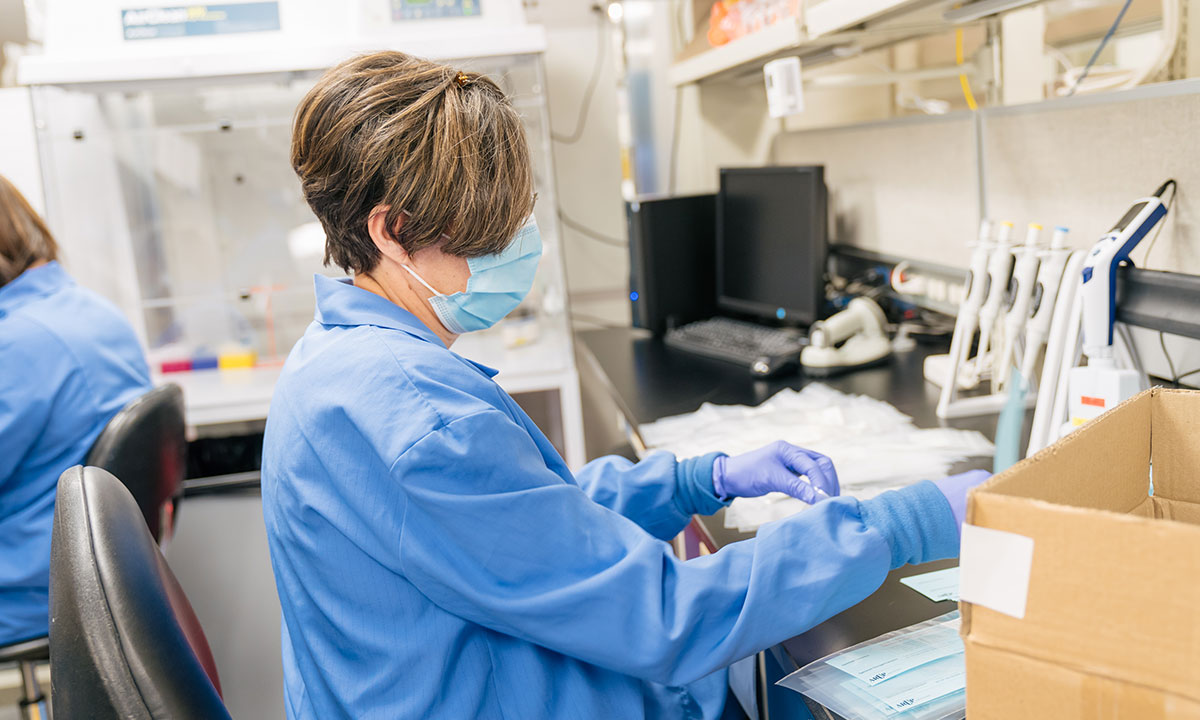
The Reagent Lab typically focuses on providing some 2,000 reagents for all of ARUP’s different labs. But with shortages and uncertainties regarding supplies, the lab took on the additional role of manually producing test collection kits, producing about 40,000 kits a week.
In March, when the COVID-19 pandemic was gaining traction in the United States, few knew what a laboratory testing collection kit included or what reagents were—until national headlines started shouting about shortages of these items.
It became clear that without collection kits, testing would not happen. ARUP’s Reagent Lab, in collaboration with the Research and Development (R&D) team, had to figure out a way to break through bottlenecks to enable more molecular testing for COVID-19.
Along with a shortage of the nasopharyngeal swabs needed to collect specimens, there was a shortage of media necessary to transport specimens in test collection kits. Transport medium is formulated to preserve specimens from the time they are collected until they arrive in the laboratory. Different media formulas are required, depending on which organisms the specimens are suspected to contain.

The Reagent Lab and R&D collaborated to develop two types of new transport media when the world’s sole supplier could not meet the demand due to COVID-19 testing. Media must be included in each test collection kit to preserve specimens from the time they are collected to the time they arrive in the laboratory.
The demand for COVID-19 testing grew rapidly, and soon the world’s sole supplier of the universal transport media (UTM) used in COVID-19 collection kits could not meet global demand.
ARUP reagent technologists worked with the ARUP R&D team to develop saline transport media for COVID-19 testing. “We basically came up with this formula, and it has kept our COVID-19 testing afloat,” said Lincoln Hirayama, who manages the Reagent Lab’s team of 60 staff members.
However, ARUP still needed transport media that would be effective for all the tests that had previously relied on UTM (approximately 50 tests). ARUP testing experts collaborated, putting in long hours, to develop new media called ARUP Transport Media.
“We needed a transport media that would support the viability of various organisms,” said Rhonda Hensley, who oversees the infectious diseases laboratories. She explained that the transport media type determines the length of time and in which conditions an organism will remain protected.
They began by experimenting with the most fragile of organisms, Mycoplasma hominis, which is linked to pelvic inflammatory disease. “This was our canary in the mine,” said Hensley. “If that worked, we knew we could move forward with testing the media with other organisms.”
They needed to be sure it would work for a variety of viruses and for testing using polymerase chain reaction (PCR) methodology. The research team repeatedly compared their results with those of FDA-approved media to ensure organism viability.
Without Testing Collection Kits, Testing Doesn’t Happen
Typically, the role of the ARUP Reagent Lab is to provide some 2,000 reagent “recipes” for tests performed in more than 60 ARUP labs. Fundamental to testing, reagents catalyze chemical reactions used to detect and characterize pathogens.
However, with shortages and uncertainties regarding supplies, the lab took on the additional role of manually producing test collection kits, producing about 40,000 kits a week.

Although the work involved in assembling test collection kits can be repetitive, everyone understands how critical it is that each kit is correct. The Reagent Lab even has its own internal quality control department to ensure product efficacy.
When the shortage first hit, the Reagent Lab doubled the number of collection kits available by halving the media in each kit and manually reassembled new kits to increase the total to 80,000 kits.
“We mobilize quickly but it is challenging,” said Hirayama. “We first thought this would be short lived, but now it has become part of our daily life here in the lab.”
Employees from other labs stepped in to help with the workload. Hirayama explained that although the work can be repetitive, everyone understands how critical it is that each kit be correct. The Reagent Lab even has its own internal quality control department to ensure product efficacy.
“We’re not on the front lines, but we know each one of these collection kits is going to end up representing a patient,” said Dallin Bagley, lead technologist. “These kits are a necessary part of the testing process. Without them, testing can’t happen.”
Just a month after the escalation of COVID-19 testing, in mid-April, orders for interleukin 6 cytokine tests spiked. The test may be useful to help guide treatment of patients with COVID-19 because it can be used to identify a potentially treatable cause of a severe hyperimmune reaction that occurs in some patients.
To help meet demand for the test, the Reagent Lab had to scramble to provide all the controls and reagents for up to 1,000 cytokine tests a week. Some employees were working 14 days straight to keep on top of this demand.
Hirayama recalled one long day when ARUP had just opened up for national COVID-19 testing. They had to assemble and supply media for 12,000 kits and meet a 4 p.m. deadline for pickup. “Everyone pitched in and after that last box was backed, a huge cheer went up.”
















