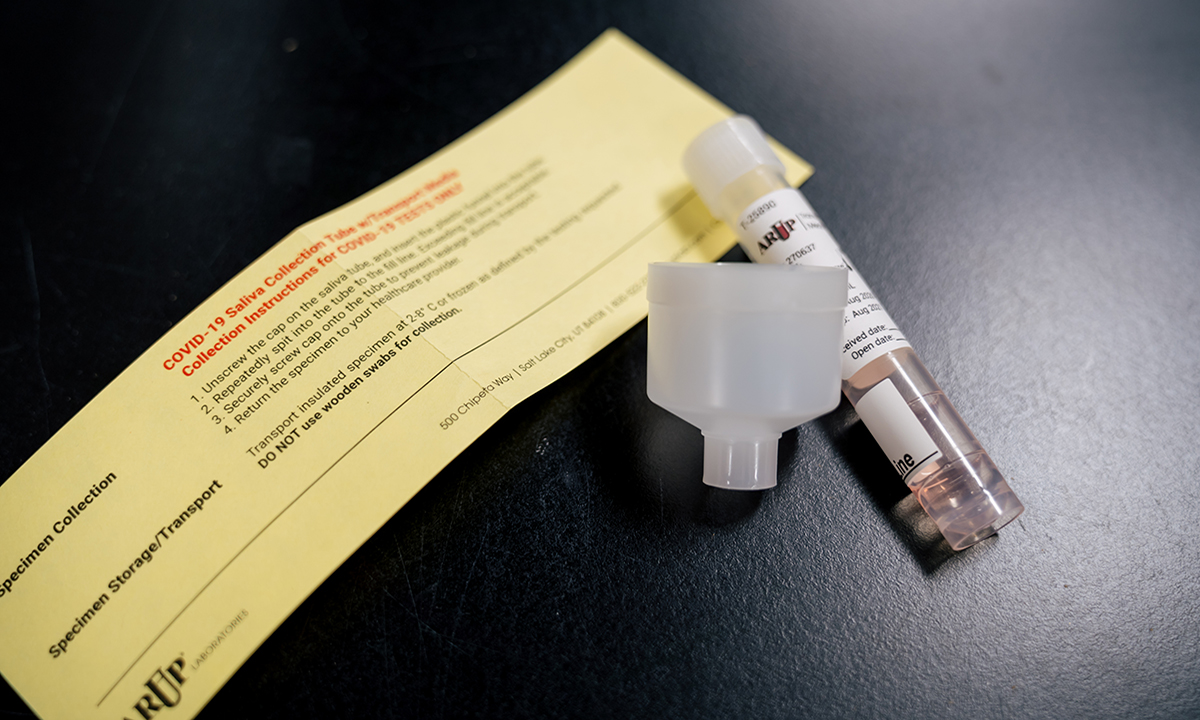
ARUP requires clients to use ARUP kits for saliva collection to optimize COVID-19 testing turnaround times on its high-throughput instrumentation.
SALT LAKE CITY – ARUP Laboratories today announced that it now offers testing to detect the virus that causes COVID-19 using saliva specimens.
The molecular diagnostic test is available with preapproval to ARUP clients nationwide. COVID-19 testing on saliva specimens offers significant benefits to both individuals and healthcare providers. Spitting into a funnel attached to the top of a collection tube is less invasive than a deep nasal swab performed by a healthcare provider. Providers benefit because they face less risk of exposure by infected individuals who cough or sneeze after enduring a nasopharyngeal swab.
ARUP will perform the test only on saliva specimens collected using an ARUP collection kit with a healthcare provider present. ARUP validated the test using its kit on high-throughput instruments, and cannot perform it on other saliva specimens without jeopardizing quick turnaround times, said Adam Barker, PhD, director of ARUP’s new COVID-19 Rapid Response Lab.
“Offering accurate, high-quality COVID-19 testing and delivering results quickly to as many patients as possible is part of our commitment to excellent patient care,” Barker said.
ARUP moved quickly to offer COVID-19 saliva testing after researchers at ARUP and University of Utah Health found self-collected saliva and nasopharyngeal swabs collected by healthcare providers are equally effective for detecting SARS-CoV-2, the virus that causes COVID-19. Their study, published in the Journal of Clinical Microbiology, is one of the largest specimen-type comparisons to date.
U of U Health began offering ARUP’s COVID-19 saliva test on Sept. 1, and is using this specimen source to test for COVID-19 infection in most clinical settings. ARUP is a nonprofit enterprise of the U of U and its Department of Pathology. In addition to performing all testing for U of U Health, ARUP has thousands of hospital and health-system clients nationwide.
“We’re pleased to be one of the first labs to offer a COVID-19 saliva test that makes high-quality testing easier for patients and for providers,’ said ARUP CEO Sherrie L. Perkins, MD, PhD. “ARUP remains committed to research, development and performance of tests that improve diagnosis of COVID-19 and all other diseases.”
In addition to COVID-19 molecular diagnostic testing, ARUP offers two IgG antibody tests to detect previous exposure to SARS-CoV-2. It also offers numerous tests that help guide treatment of the virus.
About ARUP Laboratories
Founded in 1984, ARUP Laboratories is a leading national reference laboratory and a nonprofit enterprise of the University of Utah and its Department of Pathology. ARUP offers more than 3,000 tests and test combinations, ranging from routine screening tests to esoteric molecular and genetic assays. ARUP serves clients across the United States, including many of the nation’s top university teaching hospitals and children’s hospitals, as well as multihospital groups, major commercial laboratories, group purchasing organizations, military and other government facilities, and major clinics. In addition, ARUP is a worldwide leader in innovative laboratory research and development, led by the efforts of the ARUP Institute for Clinical and Experimental Pathology®. ARUP is ISO 15189 CAP accredited.
ARUP Media Contact
Lisa Carricaburu, 801-541-5041, lisa.carricaburu@aruplab.com
















