ARUP medical directors will present at the 2024 Association of Medical Laboratory Immunologists (AMLI) Annual Meeting on emerging topics in the field.
The new Sherrie Perkins Research and Innovation Collaboration Grant, designed to foster collaboration in healthcare, will fund cutting-edge research in lab medicine that could impact patient care.
ARUP’s chief medical officer urged the House Committee on Ways and Means to consider oversight structures that both protect the public health and support innovation in healthcare.
ARUP Laboratories has been chosen as the site for a House Ways and Means Committee Field Hearing on July 12. Topics include medical innovation, healthcare access, and economic prosperity.
ARUP’s mission to continually improve patient care will carry the company into the future and drive further advancement of groundbreaking technologies and innovative solutions.
ARUP experts will present at ASM Microbe 2024 on emerging topics in infectious disease, including parasitology, mycobacterial infections, and more.
The inaugural event created connections among future innovators who look to solve many of healthcare’s biggest challenges.
This issue highlights milestones in ARUP’s history, gives a glimpse into the company’s future, and includes a special feature: a video of ARUP founders and current leaders reminiscing and celebrating.
ARUP’s new test, Alzheimer’s Disease Markers, CSF, will pave the way for more assays that help detect the disease soon enough to try therapy to slow its progression.
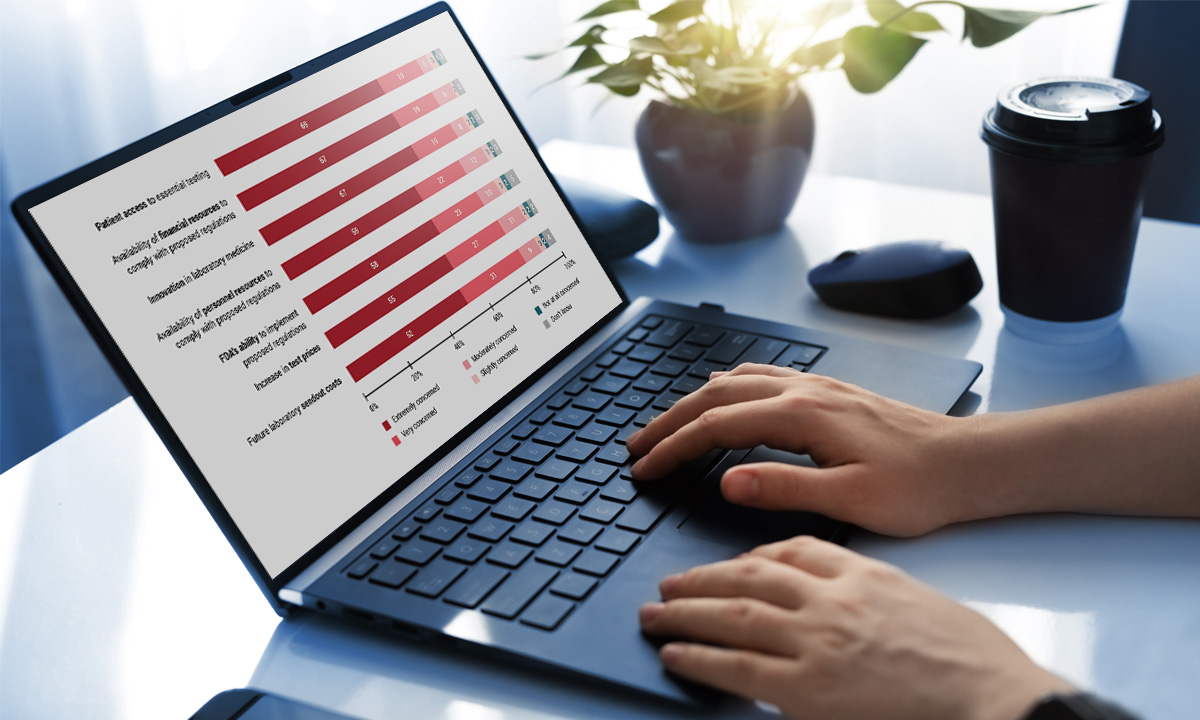
Nearly 85% of respondents to an ARUP survey believe the FDA’s proposed rule to regulate lab-developed tests will negatively impact their labs. Only 3% believe they have the financial resources to
ARUP is committed to continue offering quality, esoteric testing that can aid patients with rare diseases on their often difficult diagnostic journeys.
MetaCensus is the first open-access data repository built for peer-review and meta-analysis. The tool aims to break down stakeholder silos to quickly achieve scientific consensus.