ARUP Blood Services has aligned its donor eligibility assessment processes to follow recent U.S. Food and Drug Administration (FDA) guideline updates, and the changes will take effect on July 18, 2023. |
July 5, 2023 |
ARUP Blood Services Implements Changes in Response to New FDA Guidelines on Blood Donor The FDA now recommends the same assessment questions for all donors, regardless of gender or sexual orientation. ARUP Blood Services is one of the first donor centers to update processes in response. |
ARUP’s new test is the first companion diagnostic immunoassay for a gene therapy product to receive FDA approval. FDA has issued simultaneous approval of ROCTAVIAN™ (valoctocogene roxaparvovec-rvox), BioMarin’s gene therapy for severe hemophilia A. |
June 29, 2023 | ARUP Laboratories has received FDA approval for its companion diagnostic immunoassay, AAV5 DetectCDx™, which helps determine patient eligibility for a new gene therapy for severe hemophilia A. |
|
April 12, 2023 |
ARUP Laboratories Chooses Gestalt’s PathFlow Solution for Its Strategic ARUP and Gestalt Diagnostics announced a partnership under which ARUP will adopt Gestalt’s PathFlow® platform as a foundation for future digital pathology initiatives. |
Medical laboratory professionals such as this pair at work at ARUP are in high demand. The U.S. Bureau of Labor Statistics estimated that nearly 26,000 more of these highly trained specialists will be needed each year through 2030. |
January 10, 2023 |
Advanced Practice Clinical Laboratory Training Center at ARUP Secures $3 Million in Federal Efforts to train more medical laboratory scientists have been greatly boosted by $3 million in federal funds for a clinical lab training center that ARUP will build with the University of Utah. |
The U.S. Food and Drug Administration (FDA) has updated its blood donation guidelines to remove a former ban related to concern about variant Creutzfeldt-Jakob disease (vCJD) transmission. |
January 9, 2023 |
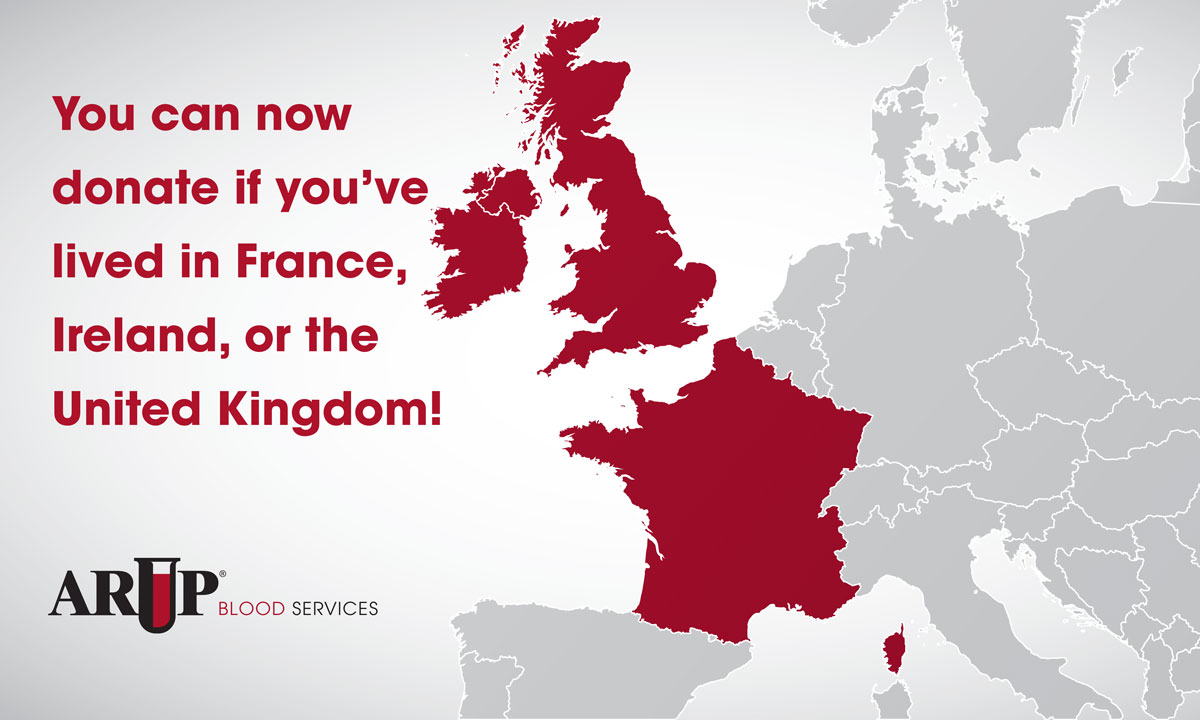
Individuals Who Lived and Worked in Parts of Europe Now Eligible to Donate Blood The U.S. Food and Drug Administration (FDA) has updated its guidelines to allow individuals who lived or worked in the United Kingdom, France, or Ireland to donate blood and platelets. |
|
December 1, 2022 |
ARUP Honored With Utah Business Magazine’s Best Companies To Work For Award ARUP’s culture of caring has earned it Utah Business magazine’s Best Companies To Work For Award for a fifth consecutive year. |