ARUP Consult, a free source of expert guidance in laboratory testing, has released new resources on newborn drug screening and noninvasive prenatal testing.
ARUP Blood Services is giving out concert tickets and National Parks annual passes to blood and platelet donors all summer.
ARUP Healthcare Advisory Services experts will hold a PLUGS presummit workshop on building successful lab stewardship programs, which can help manage costs and optimize value-based patient care.
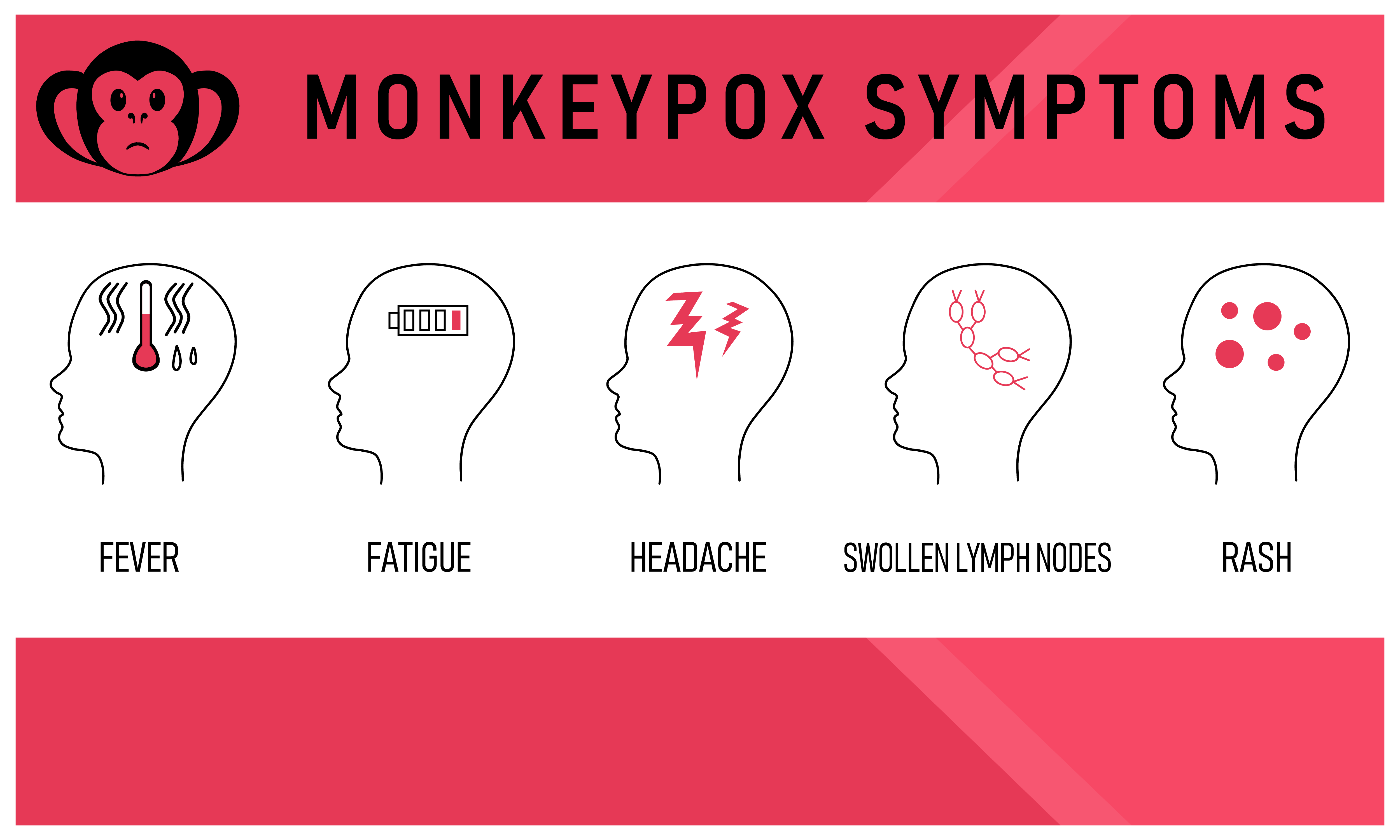
As of May 23, multiple cases of monkeypox, a rare zoonotic infection endemic to Central and West African countries, had either been confirmed or were being investigated in at least six U.S. states.
Noninvasive prenatal testing (NIPT) provides critical information to expectant parents. ARUP has updated its test offerings to include the most sensitive and specific alternatives available.
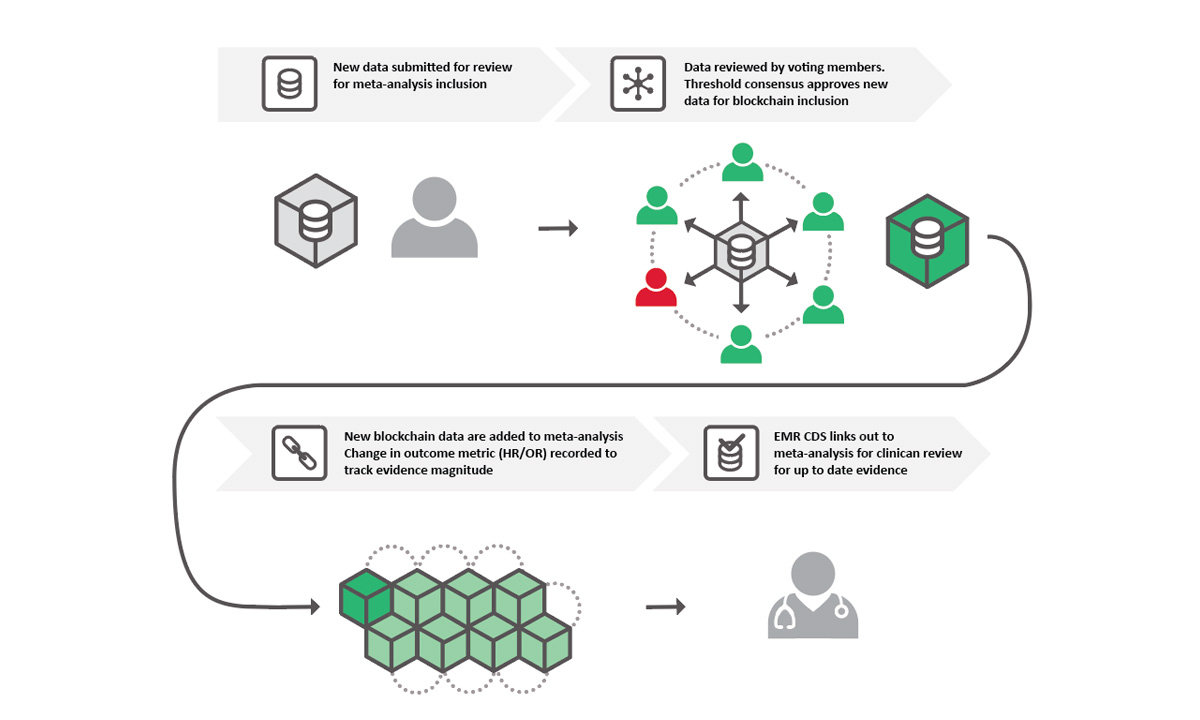
Two members of the ARUP Healthcare Advisory Services team have built the basis for an open access database that could change the way researchers perform meta-analyses.
Society's growing and changing understanding of how race, ethnicity, or ancestry (REA) is experienced and interpreted is prompting many clinical labs to reexamine practices related to REA data.
How about some dog kisses? ARUP offers dog therapy to employees to improve mental health, reduce stress, and enhance well-being.
Jonathan Genzen, MD, PhD, has been named chief medical officer and Adam Barker, PhD, has been named chief operations officer as part of a realignment of executive responsibilities.
ARUP study finds pharmacogenetic testing to evaluate drug-gene interactions improves patient outcomes and patients' confidence in overall care.
Jenna Przybyla came to ARUP for a job and found a career in laboratory science. Now, she makes a difference in patients’ lives every day. Learn how at ARUP’s Career Fair on May 13.
Samuel Rivera Aguilar’s path to becoming the University of Utah’s 2022 commencement student speaker is long and winding.