Allison Carey, MD, PhD, ARUP medical director of Hematopathology, was one of six U faculty members recognized for research excellence and invited to share their stories at the Vitae 2022 symposium.
ARUP Consult, a free source of expert guidance in laboratory testing, has updated resources on testing for bariatric surgery, respiratory viruses, and alpha-1-antitrypsin deficiency.
As respiratory viruses cocirculate this season, laboratory testing is increasingly essential to inform treatment of children, older adults, and other vulnerable populations.
The Fall 2022 edition of Magnify: The Art and Science of Diagnostic Medicine, shares how ARUP has applied what it learned from COVID-19 to improve operations, services, and, ultimately, patient care.
Benjamin Bradley, MD, PhD, joined ARUP’s team of medical directors amid the monkeypox outbreak. Now, his new research aims to help labs improve testing.
Based on data from 21,916 participants in 33 studies, the Association for the Advancement of Blood and Biotherapies (AABB) has issued guidelines for use of convalescent plasma in COVID-19 treatment.
The National Institute of Allergy and Infectious Diseases has awarded Medical Director Allison Carey, MD, PhD, $1.5 million over five years to fund her groundbreaking research.
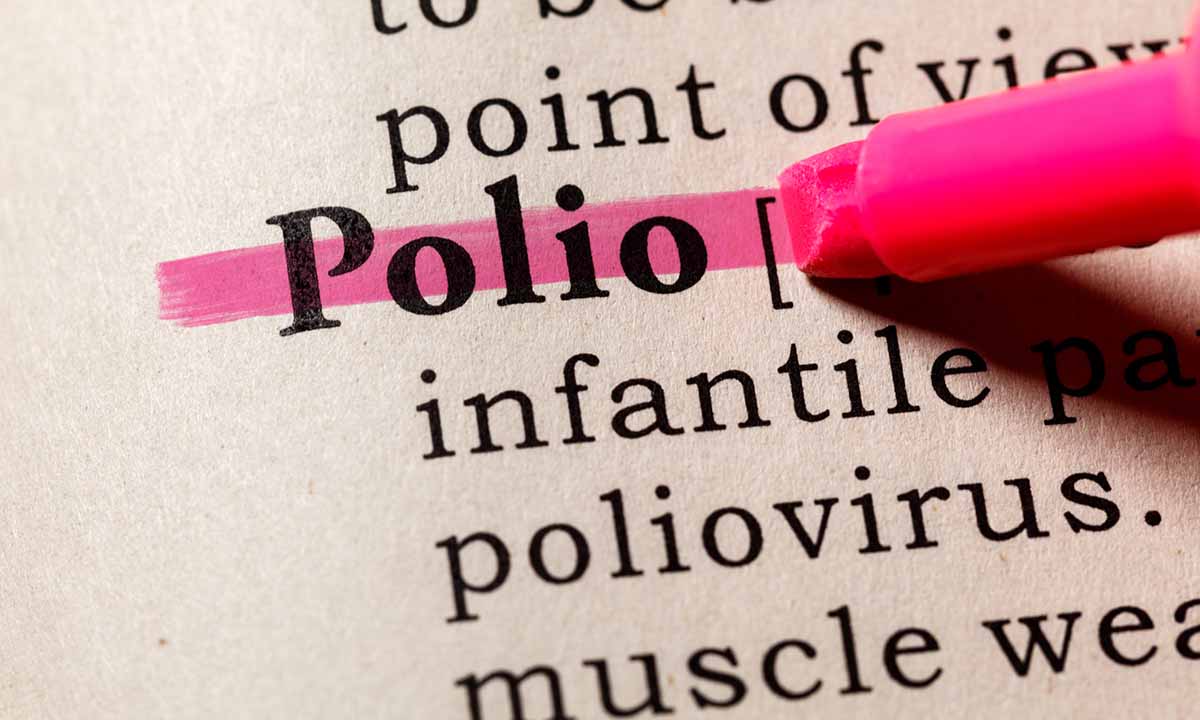
ARUP Consult has released several new and updated resources for clinicians, including testing topics on kidney evaluation, gestational trophoblastic disease, and poliovirus.
The CDC recently confirmed the first U.S. case of polio since 2013, which has raised concerns about the virus. New ARUP Consult resources inform clinicians about lab testing for poliovirus.
A new study for which ARUP provided COVID-19 testing and expertise found that emergency department workers early in the pandemic faced the highest risk of infection from community spread.
ARUP Consult, a free source of expert guidance in laboratory testing, offers valuable information on relevant topics from monkeypox to HLA testing and more.
ARUP has launched a new, in-house test to detect the DNA of orthopoxviruses, including monkeypox.