The National Institute of Allergy and Infectious Diseases has awarded Medical Director Allison Carey, MD, PhD, $1.5 million over five years to fund her groundbreaking research.
A new study for which ARUP provided COVID-19 testing and expertise found that emergency department workers early in the pandemic faced the highest risk of infection from community spread.
The Summer 2022 edition of Magnify, now online, features a look at the challenges of autoimmune neurologic testing, our experts in this area, and recent awards, and catches up with a former fellow.
ARUP medical directors and scientists will share their research and expertise in areas like lab stewardship, biochemical genetics, and neonatal drug testing at AACC’s 2022 annual meeting in Chicago.
Scientist Michael T. Pyne shares ARUP’s challenges and process of developing a next generation sequencing (NGS) test for HIV-1 antiretroviral drug resistance.
ARUP experts say that the VALID Act, which would require FDA approval of all lab-developed tests, would add administrative and financial burdens to an already safe and effective validation process.
ARUP experts are to give six talks and poster presentations at the flagship annual meeting of the American Society for Microbiology (ASM) in Washington, D.C.
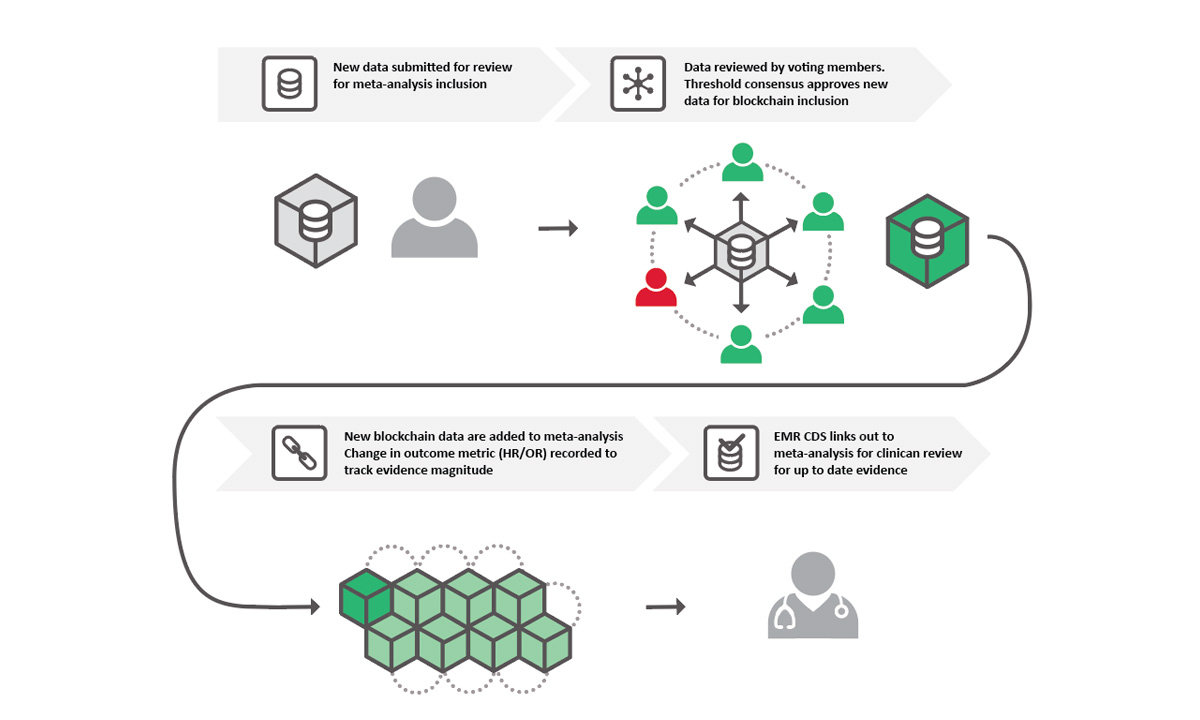
Two members of the ARUP Healthcare Advisory Services team have built the basis for an open access database that could change the way researchers perform meta-analyses.
Society's growing and changing understanding of how race, ethnicity, or ancestry (REA) is experienced and interpreted is prompting many clinical labs to reexamine practices related to REA data.
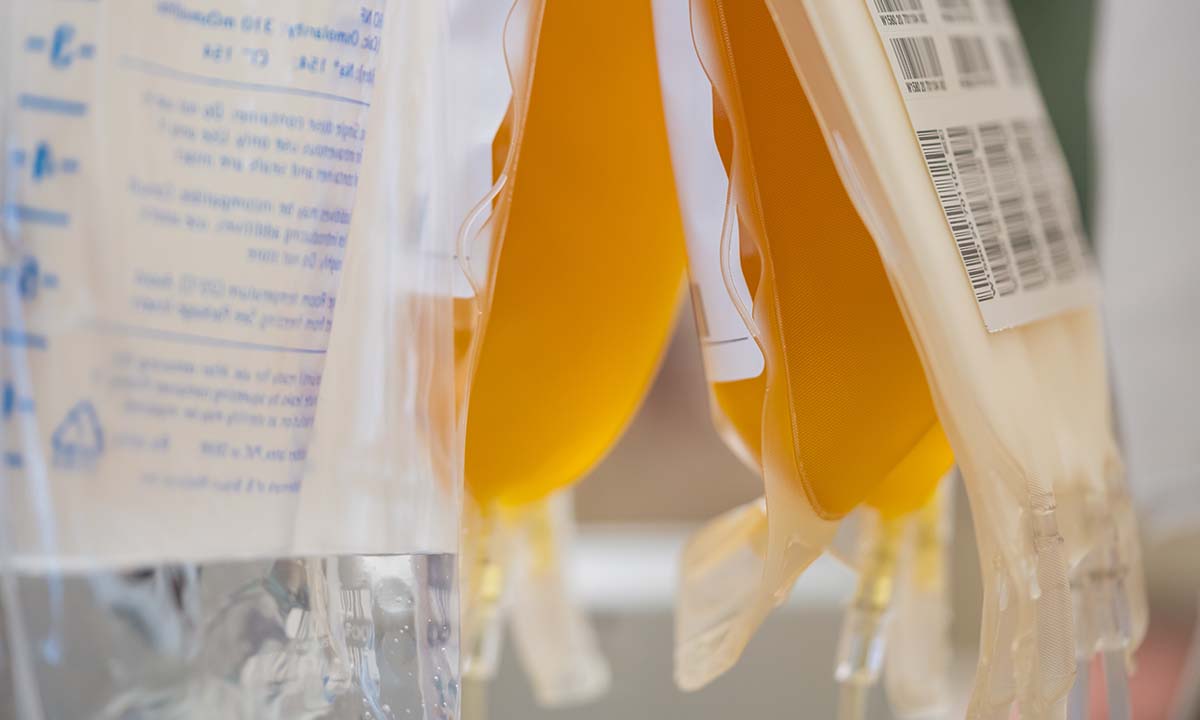
ARUP was among a few blood centers that contributed COVID-19 convalescent plasma (CCP) to a national clinical trial that found a >50% reduction in risk of hospitalization with early CCP treatment.
ARUP President and Chief Medical Officer Tracy George, MD, wants to establish a multidisciplinary, multinational network of centers focused on mast cell diagnosis, therapy, and education.
Researchers at ARUP Laboratories made significant contributions to the advancement of laboratory medicine in 2021 as coauthors of more than 150 peer-reviewed articles.