The ARUP Consult® Fragile X (FMR1)-Associated Disorders topic offers information for providers from numerous medical specialties because of the variety of conditions associated with the FMR1 gene.
The ARUP Consult Laboratory Testing for Developmental Delay, Intellectual Disability, and Autism Spectrum Disorder topic now includes exome and genome sequencing as a first- or second-tier test.
Join ARUP in kickstarting Lab Week with a curated selection of podcasts, articles, and lectures that emphasize the important role medical lab professionals play in improving and saving patient lives.
ARUP medical directors and experts will be present to discuss the latest developments in neurology laboratory testing at the upcoming American Academy of Neurology (AAN) Annual Meeting.
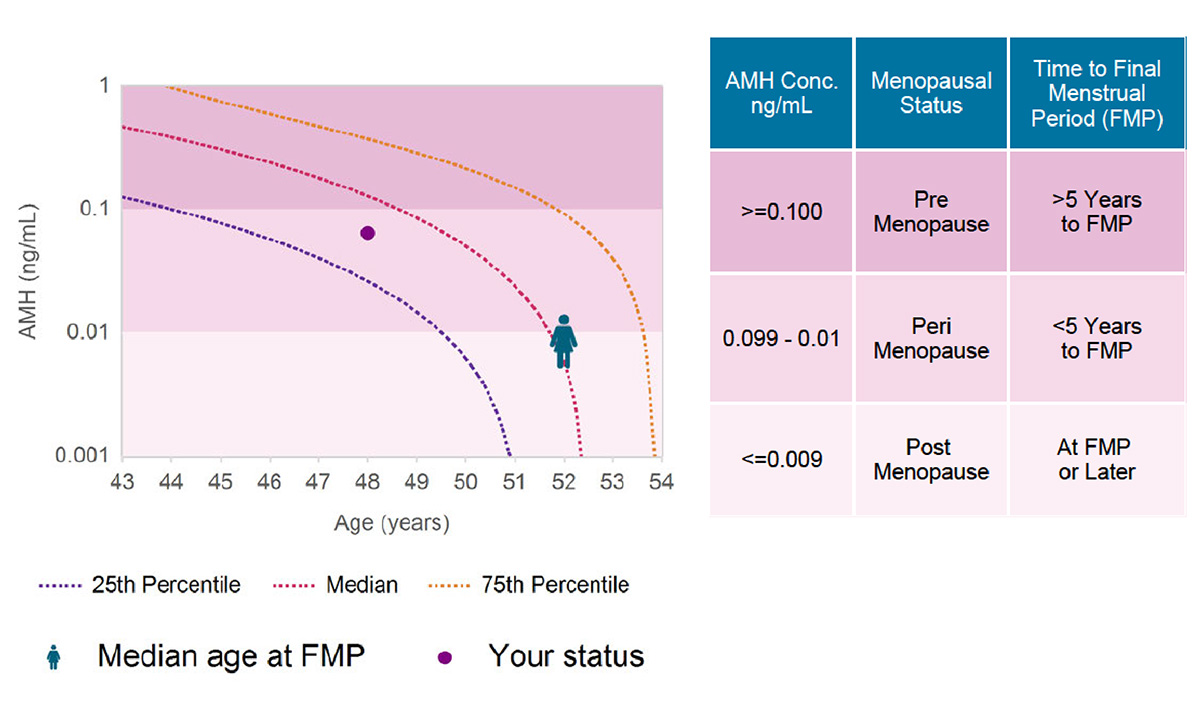
The MenoCheck test helps providers treat women who are experiencing menopausal transition and manage the health risks associated with decreased estrogen production.
ARUP’s new lupus anticoagulant panel neutralizes several interfering anticoagulants to limit their impact on test results and reduce the likelihood of false-negative and false-positive results.

Four different algorithms to lead clinicians through the correct approach to thyroid testing are available on arupconsult.com.
Lab testing is key to thyroid disease diagnosis and proper treatment but can be challenging. ARUP Consult’s thyroid disease testing resources are divided into easy-to-navigate topics and algorithms.
Next generation sequencing provides a more rigorous and sensitive method to identify drug-resistant variants of cytomegalovirus, which enables earlier detection and more effective treatment.
ARUP has gained a Conformité Européenne (CE) mark for AAV5 DetectCDx™ single-site use. The test will aid in determining the eligibility of non-U.S. patients for a new hemophilia A gene therapy.
The newest edition highlights innovations that keep ARUP at the forefront of toxicology testing. Also featured: ARUP’s expanded capacity for cytogenetics testing.
A platform clients use to reduce testing waste, lower cost per test, and improve patient safety has won the prestigious Choosing Wisely Champion Award from the American Society for Clinical Pathology.
ARUP Consult, a free source of expert guidance in laboratory testing, has released new resources on testing for polycystic ovary syndrome and autoimmune encephalitis.